In vitro
In vitro fertilisation (IVF) consists in fertilisation of an egg (eggs) in laboratory setting, and transfer of the fertilised egg into the uterus.
When in vitro procedure is used?
In vitro fertilisation provides a chance to have children when other methods of treating infertility failed, or it is known in advance that they would be unsuccessful.
IVF (in vitro fertilisation) is the most effective of all the methods of treating infertility, and it has been used successfully for over 30 years. In 2010, Robert Edwards received the most prestigious award, the Nobel prize in medicine, for developing this procedure.
Indications for in vitro procedure
Listed below are the indications recommended by the Polish Association of Reproductive Medicine, Section of Fertility and Infertility, and by the Section of Andrology of the Polish Gynaecological Association. However, it should be emphasised that each case of a couple applying for in vitro fertilisation is considered individually. What factors determine the decision to have IVF?
Tubal factor:
Considering the health of the fallopian tubes, in vitro procedure is indicated for couples where women:
- Suffer from a permanent damage to the fallopian tubes, and due to expected failure, were not qualified for surgical treatment.
- Are diagnosed with impaired function of the fallopian tubes, despite their patency.
- Following a microsurgical procedure, unsuccessfully attempted to get pregnant for no more than two years.
- Expecting pregnancy may be rational only in the absence of other potential causes of infertility (low semen quality, women aged over 35 years old, endometriosis, ovulation disorders).
Infertility of unknown origin
Idiopathic infertility, i.e. when the causes of infertility are impossible to determine, can be diagnosed after two years of unsuccessful attempts to conceive. In the case of women over 35 years of age, the condition may be diagnosed earlier.
Male factor
Indications for IVF are often associated with male infertility. They include:
- Indications for intracytoplasmic sperm injection (ICSI), i.e. fertilisation of an egg with sperm in laboratory setting.
- Sperm count of under 3 million/ml.
If the sperm count is 3-15 million/ml, the management is the same as in infertility of unknown origin.
Endometriosis
Endometriosis is a disease in which cells of the uterine mucosa do not leave the organism, but are transferred inside the reproductive organs, causing formation of cysts, nodules and adhesions, as well as bleeding.
- In stage 1 and 2 endometriosis, the management is the same as in idiopathic infertility.
- In stage 3 and 4, the indications are the same as in the case of tubal factor.
Hormonal disorders after a potential insemination failure
Six cycles with stimulation of ovulation that were ineffective, or when the stimulation resulted in the development of multiple follicles.
Other indications.
- HIV and HCV carriage by the man (the woman is not infected).
- Carriage of genetic defects resulting in severe, irreversible changes in offspring (when preimplantation diagnostics help to avoid the difficult decision regarding abortion).
- Antineoplastic treatment, which is very likely to cause irreversible damage to the ovaries.
- Premature loss of ovarian function (using a donor egg).
Preparation for in vitro procedure
Preparation of a couple for an IVF is conducted to increase the likelihood of a successful procedure. It includes:
- A medical interview regarding both partners, with emphasis on the factors affecting the safety of IVF, and determination of treatment prognosis.
- A gynaecological examination including cytological tests, vaginal pH (with normal pH, cultures are not indicated).
- A semen analysis, and in the case of abnormal results, possible additional tests, such as: karyotype, CFTR mutations; AZF is not obligatory (when the sperm count is below 5 million/ml).
- An ultrasound examination of the reproductive organ.
- A detailed assessment of the ovarian reserve – evaluation of antral follicles in an ultrasound examination, hormone testing (AMH, FSH).
- Tests involving ovarian puncture (collection of eggs in short-term general intravenous anaesthesia) – complete blood count, electrolytes, APTT, blood type.
Preparation for IVF also includes tests for abnormalities found in one of the partners.
Additional tests for women:
- • anti-Hbc, Hb antigen, anti-HCV, anti-HIV 1-2, VDRL.
- • chlamydia tracheostomy (PCR),
- • toxoplasmosis, rubella, cytomegaly virus (IgM, IgG antibodies),
- • vaginal biocenosis,
Additional tests for men:
- • anti-Hbc, Hb antigen, anti-HCV, anti-HIV 1-2, VDRL,
- • cytomegaly virus (IgM, IgG antibodies),
- • blood type.
In vitro procedure – what happens?
In vitro procedure includes four stages. What are the steps of in vitro fertilisation?
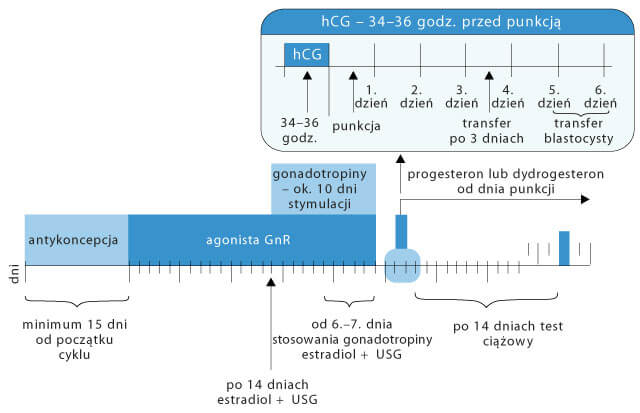
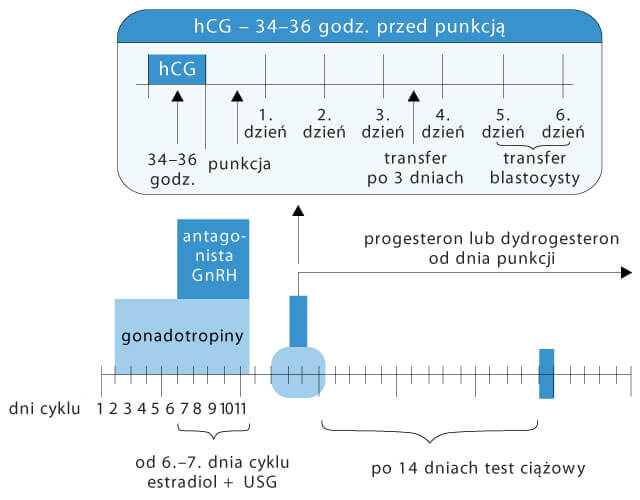
1. Stimulation of ovulation
The main goal of ovulation stimulation is to obtain more mature oocytes than in a natural cycle. It increases the chance of obtaining embryos, makes freezing the embryos possible, and thus increases the general likelihood of getting pregnant. Successful stimulation of ovulation results in a few follicles maturing in an ovary in a synchronised manner.
Presently, the following stimulation protocols are applied (listed according to the frequency of use):
- long in vitro fertilisation protocol using gonadotropin-releasing hormone agonist (GnRH-a),
- short in vitro fertilisation protocol using GnRH antagonist,
- short protocol with GnRH-a.
2. Collection of eggs
In the short-term intravenous general anaesthesia, under ultrasonographic control, a special thin needle is inserted through the vagina to the ovary, and the follicular fluid (FF) and the oocyte are collected from each follicle, one by one. It is an in-patient procedure, and it lasts about 25 minutes. After a few hours in the clinic, the patient is examined by a physician, and can be discharged home. It is important not to drive vehicles for 24 hours following the puncture (due to anaesthesia).
ICSI
Intracytoplasmic sperm injection involves injection of a single sperm into the egg. This method allows one to get pregnant even when only individual sperm cells are present in the semen (the normal level is dozens of millions).
ICSI procedure
Some of the stages overlap with those of classical in vitro fertilisation (IVF). The difference between the procedures is observed only at the laboratory stage. In classical IVF, the eggs are combined with sperm, to enable a natural fertilisation. In ICSI, a single sperm is administered to the egg by a laboratory technician.
The laboratory stage of ICSI
In the laboratory, eggs are found in the follicular fluid. After the semen is prepared, the embryologist uses, under a high magnification, a micromanipulator to aspire a normal sperm cell into a fine injection pipette (7 µm). Then the selected sperm cell is introduced into the egg.
IMSI
Intracytoplasmic Morphologically Selected Sperm Injection (IMSI) is a procedure using high magnification and high resolution to introduce a selected sperm cell with desired morphology into the cytoplasm of an egg. IMSI is based on ICSI, but involves a different method of selection of the sperm cell for injection.
Additional procedures in IVF
MESA
Micro-Epididymal Sperm Aspiration is a method that allows men with azoospermia due to obstruction of absence of vasa deferentia to have children. Under a short-lasting general intravenous anaesthesia, sperm cells are collected from the epididymis. Following their preparation, further treatment closely resembles intracytoplasmic sperm injection, i.e.:
- stimulation of ovulation,
- collection of ova,
- intracytoplasmic sperm injection,
- monitoring of embryonic development,
- embryotransfer and freezing of embryos.
This method enables men with azoospermia (due to dysfunction of the seminiferous tubules) to have children.
Laser Assisted Hatching
Laser Assisted Hatching is a system that enables to make an opening in the zona pellucida using a laser. This procedure is performed to promote implantation in the uterine wall when the zona pellucida is preventing it. The technique uses a highly focused laser beam to remove with great precision a small area of the outer shell of the embryo.
Extended observation of embryos until the blastocyst stage
It is a standard procedure in IVF to observe the development of in vitro embryos before they are transferred to the uterus on day two or three following the fertilisation (4-9 cell stadium).
When on day 3 we cannot find an embryo with potential for development, we extend the observation to day 5, i.e. to the blastocyst stage.
Preservation (freezing) of eggs and embryos
Vitrification is transformation from a liquid state into solid state without ice crystal formation. It consists in a rapid cooling of the egg/embryo in cryoprotectant at 20000 degrees Celsius per minute to under -150°C.
Time-laps system (a system for continuous embryo monitoring)
It is a system for continuous in vitro embryo development monitoring (in-incubator). It enables archiving and analysis of images from selected stages in embryo development.
The Time – laps system involves placing a microscope camera directly in the incubator when the embryo is stored. This allows us to:
- continuously monitor the development of embryos,
- monitor the embryos without removing them from the incubator.
The Time – laps system monitors the development of each embryo individually, from the moment of fertilisation until the day of transfer. The images are taken at high frequency, even every five minutes. The system uses these images to create a film that allows to observe and emphasise the processes in the embryo development that are normally too slow to see.
This observation method does not require removing the embryos from the incubator, which offers an advantage over the traditional monitoring method. The embryos can remain in an optimal environment, and are not exposed to adverse changes in pH, or potentially harmful effects of intense light.
Indications for Time – laps
The constant embryo monitoring system may significantly improve selection of the best embryos for transfer, thus increasing the chance of giving birth to a healthy child, especially in couples:
- who experienced in vitro failures;
- who obtain a large number of embryos with identical morphological characteristics (preventing the selection of the ideal embryo for transfer).
The method that facilitates identification of embryos with the greatest developmental potential, even at early stages, may also help to reduce the number of embryos transferred to the uterus, lowering the rate of multiple pregnancies following in vitro procedures.
